Different Methods to Improve the Monitoring of Noninvasive Respiratory Support of Patients with Severe Pneumonia/ARDS Due to COVID-19: An Update
Abstract
:1. Introduction
2. Physio-Pathological Differences and Similarities between COVID-19 and Non-COVID-19 ARDS
2.1. Non-COVID-19 ARDS
2.2. COVID-19 ARDS
3. The Pathophysiology of Severe Pneumonia/ARDS Due to COVID-19: Correlation from Lung CT Scan and Histology Findings with Clinical Phenotypes
4. Noninvasive Measurement of Compliance: Role of the Forced Oscillation Technique (FOT)
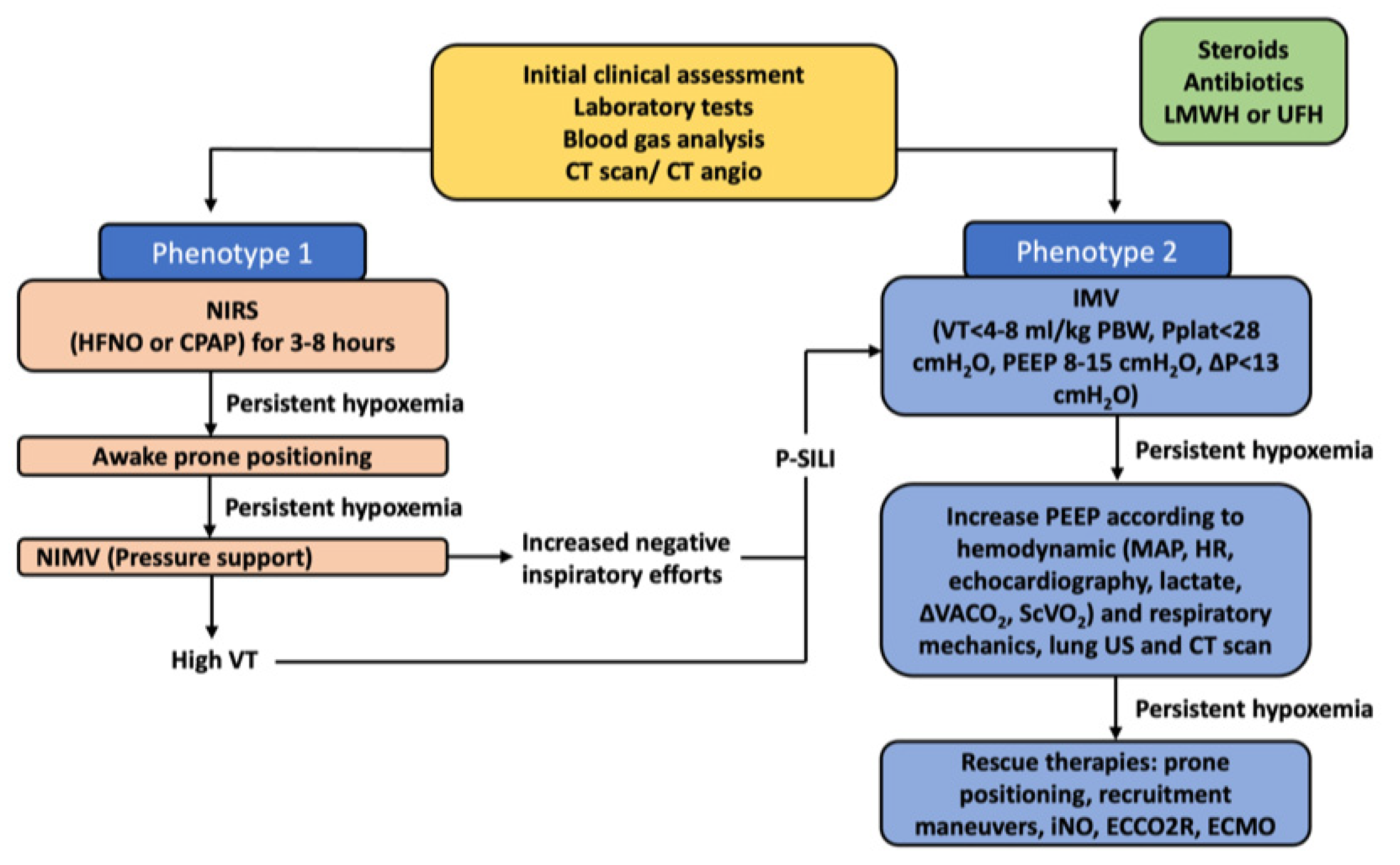
5. Noninvasive Respiratory Support in Severe COVID-19
6. Physiological Parameters Monitoring during Noninvasive Support for Acute Respiratory Failure Due to Severe COVID-19
7. Summary and New Perspectives
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- WHO. WHO Coronavirus (COVID-19) Dashboard. 2021. Available online: https://covid19.who.int (accessed on 16 February 2022).
- Hermann, M.; Laxar, D.; Krall, C.; Hafner, C.; Herzog, O.; Kimberger, O. Duration of invasive mechanical ventilation prior to extracorporeal membrane oxygenation is not associated with survival in acute respiratory distress syndrome caused by coronavirus disease 2019. Ann. Intensive Care 2022, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Ball, L.; Barisione, E.; Mastracci, L.; Campora, M.; Costa, D.; Robba, C.; Battaglini, D.; Micali, M.; Costantino, F.; Cittadini, G.; et al. Extension of Collagen Deposition in COVID-19 Post Mortem Lung Samples and Computed Tomography Analysis Findings. Int. J. Mol. Sci. 2021, 22, 7498. [Google Scholar] [CrossRef]
- Baratella, E.; Ruaro, B.; Marrocchio, C.; Starvaggi, N.; Salton, F.; Giudici, F.; Quaia, E.; Confalonieri, M.; Cova, M.A. Interstitial Lung Disease at High Resolution CT after SARS-CoV-2-Related Acute Respiratory Distress Syndrome According to Pulmonary Segmental Anatomy. J. Clin. Med. 2021, 10, 3985. [Google Scholar] [CrossRef] [PubMed]
- Radermacher, P.; Maggiore, S.M.; Mercat, A. Fifty Years of Research in ARDS. Gas Exchange in Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2017, 196, 964–984. [Google Scholar] [CrossRef]
- Petersson, J.; Glenny, R.W. Gas exchange and ventilation–perfusion relationships in the lung. Eur. Respir. J. 2014, 44, 1023–1041. [Google Scholar] [CrossRef] [Green Version]
- Botta, M.; Tsonas, A.M.; Pillay, J.; Boers, L.S.; Algera, A.G.; Bos, L.D.J.; Dongelmans, D.A.; Hollmann, M.W.; Horn, J.; Vlaar, A.P.J.; et al. Ventilation management and clinical outcomes in invasively ventilated patients with COVID-19 (PRoVENT-COVID): A national, multicentre, observational cohort study. Lancet Respir. Med. 2021, 9, 139–148. [Google Scholar] [CrossRef]
- Rocco, P.R.; Pelosi, P. Pulmonary and extrapulmonary acute respiratory distress syndrome: Myth or reality? Curr. Opin. Crit. Care 2008, 14, 50–55. [Google Scholar] [CrossRef]
- Meduri, G.U.; Annane, D.; Confalonieri, M.; Chrousos, G.P.; Rochwerg, B.; Busby, A.; Ruaro, B.; Meibohm, B. Pharmacological principles guiding prolonged glucocorticoid treatment in ARDS. Intensiv. Care Med. 2020, 46, 2284–2296. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Caironi, P.; Pelosi, P.; Goodman, L.R. What Has Computed Tomography Taught Us about the Acute Respiratory Distress Syndrome? Am. J. Respir. Crit. Care Med. 2001, 164, 1701–1711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelosi, P.; D’Andrea, L.; Vitale, G.; Pesenti, A.; Gattinoni, L. Vertical gradient of regional lung inflation in adult respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1994, 149, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Chiumello, D.; Caironi, P.; Busana, M.; Romitti, F.; Brazzi, L.; Camporota, L. COVID-19 pneumonia: Different respiratory treatments for different phenotypes? Intensive Care Med. 2020, 46, 1099–1102. [Google Scholar] [CrossRef] [PubMed]
- Dakin, J.; Jones, A.T.; Hansell, D.M.; Hoffman, E.A.; Evans, T.W. Changes in lung composition and regional perfusion and tissue distribution in patients with ARDS. Respirology 2011, 16, 1265–1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salton, F.; Confalonieri, P.; Meduri, G.U.; Santus, P.; Harari, S.; Scala, R.; Lanini, S.; Vertui, V.; Oggionni, T.; Caminati, A.; et al. Prolonged Low-Dose Methylprednisolone in Patients With Severe COVID-19 Pneumonia. Open Forum Infect. Dis. 2020, 7. [Google Scholar] [CrossRef]
- Ruaro, B.; Salton, F.; Braga, L.; Wade, B.; Confalonieri, P.; Volpe, M.C.; Baratella, E.; Maiocchi, S.; Confalonieri, M. The History and Mystery of Alveolar Epithelial Type II Cells: Focus on Their Physiologic and Pathologic Role in Lung. Int. J. Mol. Sci. 2021, 22, 2566. [Google Scholar] [CrossRef]
- Orlandi, M.; Landini, N.; Sambataro, G.; Nardi, C.; Tofani, L.; Bruni, C.; Randone, S.B.; Blagojevic, J.; Melchiorre, D.; Hughes, M.; et al. The role of chest CT in deciphering interstitial lung involvement: Systemic sclerosis versus COVID-19. Rheumatology 2021. ahead of print. [Google Scholar] [CrossRef]
- Ball, L.; Robba, C.; Herrmann, J.; Gerard, S.E.; Xin, Y.; Mandelli, M.; Battaglini, D.; Brunetti, I.; Minetti, G.; Seitun, S.; et al. Lung distribution of gas and blood volume in critically ill COVID-19 patients: A quantitative dual-energy computed tomography study. Crit. Care 2021, 25, 214. [Google Scholar] [CrossRef] [PubMed]
- Robba, C.; Battaglini, D.; Ball, L.; Pelosi, P.; Rocco, P.R.M. Ten things you need to know about intensive care unit management of mechanically ventilated patients with COVID-19. Expert Rev. Respir. Med. 2021, 15, 1293–1302. [Google Scholar] [CrossRef]
- Malbouisson, L.M.; Muller, J.C.; Constantin, J.M.; Lu, Q.; Puybasset, L.; Rouby, J.J. Computed Tomography Assessment of Positive End-expiratory Pressure-induced Alveolar Recruitment in Patients with Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2001, 163, 1444–1450. [Google Scholar] [CrossRef] [PubMed]
- Ball, L.; Robba, C.; Maiello, L.; Herrmann, J.; Gerard, S.E.; Xin, Y.; Battaglini, D.; Brunetti, I.; Minetti, G.; Seitun, S.; et al. Computed tomography assessment of PEEP-induced alveolar recruitment in patients with severe COVID-19 pneumonia. Crit. Care 2021, 25, 81. [Google Scholar] [CrossRef] [PubMed]
- Robba, C.; Battaglini, D.; Ball, L.; Patroniti, N.; Loconte, M.; Brunetti, I.; Vena, A.; Giacobbe, D.R.; Bassetti, M.; Rocco, P.R.M.; et al. Distinct phenotypes require distinct respiratory management strategies in severe COVID-19. Respir. Physiol. Neurobiol. 2020, 279, 103455. [Google Scholar] [CrossRef]
- Torregiani, C.; Veneroni, C.; Confalonieri, P.; Citton, C.; Salton, F.; Jaber, M.; Confalonieri, M.; Dellaca’, R. Monitoring lung mechanics by oscillometry in COVID 19 ARDS receiving non-invasive ventilation: A pilot study. Eur. Respir. J. 2021, 58 (Suppl 65), PA1771. [Google Scholar] [CrossRef]
- Tonelli, R.; Marchioni, A.; Tabbì, L.; Fantini, R.; Busani, S.; Castaniere, I.; Andrisani, D.; Gozzi, F.; Bruzzi, G.; Manicardi, L.; et al. Spontaneous Breathing and Evolving Phenotypes of Lung Damage in Patients with COVID-19: Review of Current Evidence and Forecast of a New Scenario. J. Clin. Med. 2021, 10, 975. [Google Scholar] [CrossRef]
- Ranjeva, S.; Pinciroli, R.; Hodell, E.; Mueller, A.; Hardin, C.C.; Thompson, B.T.; Berra, L. Identifying clinical and biochemical phenotypes in acute respiratory distress syndrome secondary to coronavirus disease-2019. EClinicalMedicine 2021, 34, 100829. [Google Scholar] [CrossRef] [PubMed]
- Baratella, E.; Crivelli, P.; Marrocchio, C.; Marco Bozzato, A.; De Vito, A.; Madeddu, G.; Saderi, L.; Confalonieri, M.; Tenaglia, L.; Assunta Cova, M. Severity of lung involvement on chest X-rays in SARS-coronavirus-2 infected patients as a possible tool to predict clinical progression: An observational retrospective analysis of the relationship between radiological, clinical, and laboratory data. J. Bras. Pneumol. 2020, 46, e20200226. [Google Scholar] [CrossRef] [PubMed]
- Borghesi, A.; Maroldi, R. COVID-19 outbreak in Italy: Experimental chest X-ray scoring system for quantifying and monitoring disease progression. Radiol. Med. 2020, 125, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.F.; Pershad, Y.; Kang, P.; Ridenour, L.; Lavon, B.; Lanclus, M.; Godon, R.; De Backer, J.; Glassberg, M.K. Altered pulmonary blood volume distribution as a biomarker for predicting outcomes in COVID-19 disease. Eur. Respir. J. 2021, 58, 2004133. [Google Scholar] [CrossRef]
- Colombi, D.; Bodini, F.C.; Petrini, M.; Maffi, G.; Morelli, N.; Milanese, G.; Silva, M.; Sverzellati, N.; Michieletti, E. Well-aerated Lung on Admitting Chest CT to Predict Adverse Outcome in COVID-19 Pneumonia. Radiology 2020, 296, E86–E96. [Google Scholar] [CrossRef] [Green Version]
- Martini, K.; Larici, A.R.; Revel, M.P.; Ghaye, B.; Sverzellati, N.; Parkar, A.P.; Snoeckx, A.; Screaton, N.; Biederer, J.; Prosch, H.; et al. COVID-19 pneumonia imaging follow-up: When and how? A proposition from ESTI and ESR. Eur. Radiol. 2021, 32, 2639–2649. [Google Scholar] [CrossRef]
- Baratella, E.; Roman-Pognuz, E.; Zerbato, V. Potential links between COVID-19-associated pulmonary aspergillosis and bronchiectasis as detected by high resolution computed tomography. Front. Biosci. 2021, 26, 1607–1612. [Google Scholar] [CrossRef]
- Han, X.; Fan, Y.; Alwalid, O.; Li, N.; Jia, X.; Yuan, M.; Li, Y.; Cao, Y.; Gu, J.; Wu, H.; et al. Six-month Follow-up Chest CT Findings after Severe COVID-19 Pneumonia. Radiology 2021, 299, E177–E186. [Google Scholar] [CrossRef]
- Caruso, D.; Guido, G.; Zerunian, M.; Polidori, T.; Lucertini, E.; Pucciarelli, F.; Polici, M.; Rucci, C.; Bracci, B.; Nicolai, M.; et al. Post-Acute Sequelae of COVID-19 Pneumonia: Six-month Chest CT Follow-up. Radiology 2021, 301, E396–E405. [Google Scholar] [CrossRef]
- Wigén, J.; Löfdahl, A.; Bjermer, L.; Elowsson Rendin, L.; Westergren-Thorsson, G. Converging pathways in pulmonary fibrosis and COVID-19—The fibrotic link to disease severity. Respir. Med. X 2020, 2, 100023. [Google Scholar] [CrossRef] [PubMed]
- Mineo, G.; Ciccarese, F.; Modolon, C.; Landini, M.P.; Valentino, M.; Zompatori, M. Post-ARDS pulmonary fibrosis in patients with H1N1 pneumonia: Role of follow-up CT. Radiol. Med. 2012, 117, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Zieleskiewicz, L.; Markarian, T.; Lopez, A.; Taguet, C.; Mohammedi, N.; Boucekine, M.; Baumstarck, K.; Besch, G.; Mathon, G.; Duclos, G.; et al. Comparative study of lung ultrasound and chest computed tomography scan in the assessment of severity of confirmed COVID-19 pneumonia. Intensive Care Med. 2020, 46, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Tung-Chen, Y.; Martí de Gracia, M.; Díez-Tascón, A.; Alonso-González, R.; Agudo-Fernández, S.; Parra-Gordo, M.L.; Ossaba-Vélez, S.; Rodríguez-Fuertes, P.; Llamas-Fuentes, R. Correlation between Chest Computed Tomography and Lung Ultrasonography in Patients with Coronavirus Disease 2019 (COVID-19). Ultrasound Med. Biol. 2020, 46, 2918–2926. [Google Scholar] [CrossRef]
- Lopes, A.J.; Mafort, T.T.; Costa, C.H.; Rufino, R.; Cássia Firmida, M.; Kirk, K.M.; Cobo, C.G.; Costa, H.d.S.B.; Cruz, C.M.B.Q.; Mogami, R. Comparison Between Lung Ultrasound and Computed Tomographic Findings in Patients With COVID-19 Pneumonia. J. Ultrasound Med. 2021, 40, 1391–1399. [Google Scholar] [CrossRef]
- Kok, B.; Schuit, F.; Lieveld, A.; Azijli, K.; Nanayakkara, P.W.; Bosch, F. Comparing lung ultrasound: Extensive versus short in COVID-19 (CLUES): A multicentre, observational study at the emergency department. BMJ Open 2021, 11, e048795. [Google Scholar] [CrossRef]
- Gutsche, H.; Lesser, T.G.; Wolfram, F.; Doenst, T. Significance of Lung Ultrasound in Patients with Suspected COVID-19 Infection at Hospital Admission. Diagnostics 2021, 11, 921. [Google Scholar] [CrossRef] [PubMed]
- Cogliati, C.; Bosch, F.; Tung-Chen, Y.; Smallwood, N.; Torres-Macho, J. Lung ultrasound in COVID-19: Insights from the frontline and research experiences. Eur. J. Intern. Med. 2021, 90, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Lerum, T.V.; Aaløkken, T.M.; Brønstad, E.; Aarli, B.; Ikdahl, E.; Lund, K.M.A.; Durheim, M.T.; Rodriguez, J.R.; Meltzer, C.; Tonby, K.; et al. Dyspnoea, lung function and CT findings 3 months after hospital admission for COVID-19. Eur. Respir. J. 2021, 57, 2003448. [Google Scholar] [CrossRef]
- Allinovi, M.; Parise, A.; Giacalone, M.; Amerio, A.; Delsante, M.; Odone, A.; Franci, A.; Gigliotti, F.; Amadasi, S.; Delmonte, D.; et al. Lung Ultrasound May Support Diagnosis and Monitoring of COVID-19 Pneumonia. Ultrasound Med. Biol. 2020, 46, 2908–2917. [Google Scholar] [CrossRef]
- Landini, N.; Orlandi, M.; Fusaro, M.; Ciet, P.; Nardi, C.; Bertolo, S.; Catalanotti, V.; Matucci-Cerinic, M.; Colagrande, S.; Morana, G. The Role of Imaging in COVID-19 Pneumonia Diagnosis and Management: Main Positions of the Experts, Key Imaging Features and Open Answers. J. Cardiovasc. Echogr. 2020, 30, S25–S30. [Google Scholar]
- Lepri, G.; Orlandi, M.; Lazzeri, C.; Bruni, C.; Hughes, M.; Bonizzoli, M.; Wang, Y.; Peris, A.; Matucci-Cerinic, M. The emerging role of lung ultrasound in COVID-19 pneumonia. Eur. J. Rheumatol. 2020, 7, S129–S133. [Google Scholar] [CrossRef] [PubMed]
- Gregorio-Hernández, R.; Escobar-Izquierdo, A.B.; Cobas-Pazos, J.; Martínez-Gimeno, A. Point-of-care lung ultrasound in three neonates with COVID-19. Eur. J. Pediatr. 2020, 179, 1279–1285. [Google Scholar] [CrossRef]
- Pivetta, E.; Goffi, A.; Tizzani, M.; Locatelli, S.M.; Porrino, G.; Losano, I.; Leone, D.; Calzolari, G.; Vesan, M.; Steri, F.; et al. Lung Ultrasonography for the Diagnosis of SARS-CoV-2 Pneumonia in the Emergency Department. Ann. Emerg. Med. 2021, 77, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Sorlini, C.; Femia, M.; Nattino, G.; Bellone, P.; Gesu, E.; Francione, P.; Paternò, M.; Grillo, P.; Ruffino, A.; Bertolini, G.; et al. The role of lung ultrasound as a frontline diagnostic tool in the era of COVID-19 outbreak. Intern. Emerg. Med. 2021, 16, 749–756. [Google Scholar] [CrossRef]
- Di Serafino, M.; Notaro, M.; Rea, G.; Iacobellis, F.; Delli Paoli, V.; Acampora, C.; Ianniello, S.; Brunese, L.; Romano, L.; Vallone, G. The lung ultrasound: Facts or artifacts? In the era of COVID-19 outbreak. Radiol. Med. 2020, 125, 738–753. [Google Scholar] [CrossRef] [PubMed]
- Zhanna Davidovna, K.; Fuad Safarova, A.; Cabello Montoya, F.E.; Vatsik-Gorodetskaya, M.V.; Yulia Leonidovna, K.; Olga Tairovna, Z.; Olga Valeryevna, A.; Rajan, R.; Al Jarallah, M.; Brady, P.A.; et al. A single-center comparative study of lung ultrasound versus chest computed tomography during the COVID-19 era. Multidiscip. Respir. Med. 2021, 16, 766. [Google Scholar] [CrossRef]
- Pivetta, E.; Girard, E.; Locascio, F.; Lupia, E.; Martin, J.D.; Stone, M. Self-Performed Lung Ultrasound for Home Monitoring of a Patient Positive for Coronavirus Disease 2019. Chest 2020, 158, e93–e97. [Google Scholar] [CrossRef]
- Brashier, B.; Salvi, S. Measuring lung function using sound waves: Role of the forced oscillation technique and impulse oscillometry system. Breathe 2015, 11, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Kaczka, D.W.; Dellaca, R.L. Oscillation Mechanics of the Respiratory System: Applications to Lung Disease. Crit. Rev. Biomed. Eng. 2011, 39, 337–359. [Google Scholar] [CrossRef] [Green Version]
- Alblooshi, A.S.; Simpson, S.J.; Stick, S.M.; Hall, G.L. The safety and feasibility of the inhaled mannitol challenge test in young children. Eur. Respir. J. 2013, 42, 1420–1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, D.; Willemse, L.; Visagie, A.; Czövek, D.; Nduru, P.; Vanker, A.; Stein, D.J.; Koen, N.; Sly, P.D.; Hantos, Z.; et al. Determinants of early-life lung function in African infants. Thorax 2017, 72, 445–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farré, R.; Gavela, E.; Rotger, M.; Ferrer, M.; Roca, J.; Navajas, D. Noninvasive assessment of respiratory resistance in severe chronic respiratory patients with nasal CPAP. Eur. Respir. J. 2000, 15, 314. [Google Scholar] [CrossRef]
- Dellacà, R.L.; Rotger, M.; Aliverti, A.; Navajas, D.; Pedotti, A.; Farré, R. Noninvasive detection of expiratory flow limitation in COPD patients during nasal CPAP. Eur. Respir. J. 2006, 27, 983–991. [Google Scholar] [CrossRef] [Green Version]
- Lorx, A.; Suki, B.; Hercsuth, M.; Szabó, B.; Pénzes, I.; Boda, K.; Hantos, Z. Airway and tissue mechanics in ventilated patients with pneumonia. Respir. Physiol. Neurobiol. 2010, 171, 101–109. [Google Scholar] [CrossRef]
- Walker, P.P.; Pompilio, P.P.; Zanaboni, P.; Bergmo, T.S.; Prikk, K.; Malinovschi, A.; Montserrat, J.M.; Middlemass, J.; Šonc, S.; Munaro, G.; et al. Telemonitoring in Chronic Obstructive Pulmonary Disease (CHROMED). A Randomized Clinical Trial. Am. J. Respir. Crit. Care Med. 2018, 198, 620–628. [Google Scholar] [CrossRef]
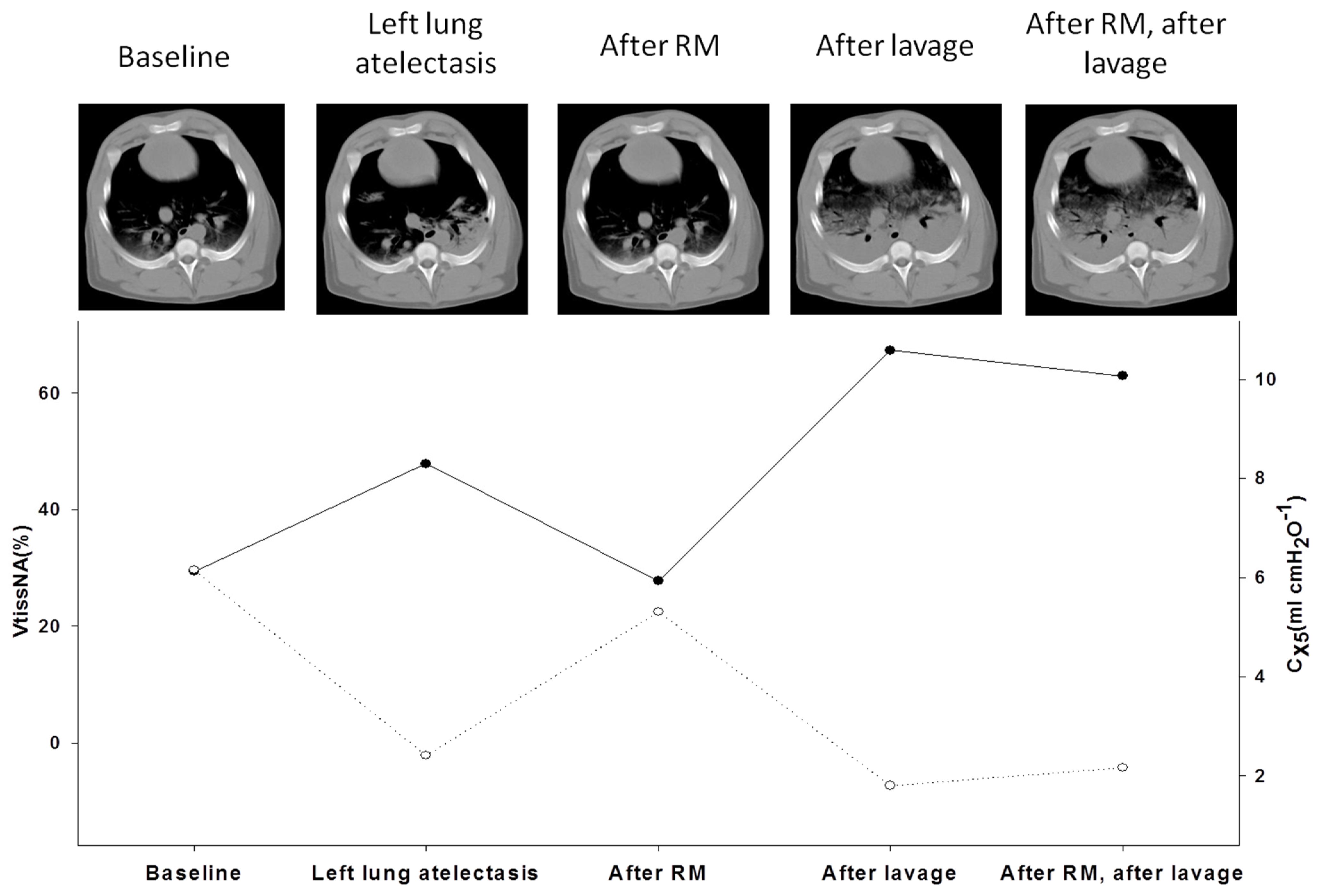
- Dellaca, R.L.; Andersson Olerud, M.; Zannin, E.; Kostic, P.; Pompilio, P.P.; Hedenstierna, G.; Pedotti, A.; Frykholm, P. Lung recruitment assessed by total respiratory system input reactance. Intensive Care Med. 2009, 35, 2164. [Google Scholar] [CrossRef]
- Wallström, L.; Veneroni, C.; Zannin, E.; Dellacà, R.L.; Sindelar, R. Forced oscillation technique for optimising PEEP in ventilated extremely preterm infants. Eur. Respir. J. 2020, 55, 1901650. [Google Scholar] [CrossRef] [PubMed]
- Dellacà, R.L.; Zannin, E.; Kostic, P.; Andersson Olerud, M.; Pompilio, P.P.; Hedenstierna, G.; Pedotti, A.; Frykholm, P. Optimisation of positive end-expiratory pressure by forced oscillation technique in a lavage model of acute lung injury. Intensive Care Med. 2011, 37, 1021. [Google Scholar] [CrossRef] [PubMed]
- Papoutsi, E.; Giannakoulis, V.G.; Xourgia, E.; Routsi, C.; Kotanidou, A.; Siempos, I.I. Effect of timing of intubation on clinical outcomes of critically ill patients with COVID-19: A systematic review and meta-analysis of non-randomized cohort studies. Crit. Care 2021, 25, 121. [Google Scholar] [CrossRef]
- Baratella, E.; Bussani, R.; Zanconati, F. Radiological-pathological signatures of patients with COVID-19-related pneumomediastinum: Is there a role for the Sonic hedgehog and Wnt5a pathways? ERJ Open Res. 2021, 7, 00346. [Google Scholar] [CrossRef] [PubMed]
- Battaglini, D.; Caiffa, S.; Gasti, G.; Ciaravolo, E.; Robba, C.; Herrmann, J.; Gerard, S.; Bassetti, M.; Pelosi, P.; Ball, L. An Experimental Pre-Post Study on the Efficacy of Respiratory Physiotherapy in Severe Critically III COVID-19 Patients. J. Clin. Med. 2021, 10, 2139. [Google Scholar] [CrossRef]
- Battaglini, D.; Robba, C.; Caiffa, S.; Ball, L.; Brunetti, I.; Loconte, M.; Giacobbe, D.R.; Vena, A.; Patroniti, N.; Bassetti, M.; et al. Chest physiotherapy: An important adjuvant in critically ill mechanically ventilated patients with COVID-19. Respir. Physiol. Neurobiol. 2020, 282, 103529. [Google Scholar] [CrossRef] [PubMed]
- Putensen, C.; Zech, S.; Wrigge, H.; Zinserling, J.; Stuber, F.; Von Spiegel, T.; Mutz, N. Long-Term Effects of Spontaneous Breathing During Ventilatory Support in Patients with Acute Lung Injury. Am. J. Respir. Crit. Care Med. 2001, 164, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Goligher, E.C.; Dres, M.; Fan, E.; Rubenfeld, G.D.; Scales, D.C.; Herridge, M.S.; Vorona, S.; Sklar, M.C.; Rittayamai, N.; Lanys, A.; et al. Mechanical Ventilation–induced Diaphragm Atrophy Strongly Impacts Clinical Outcomes. Am. J. Respir. Crit. Care Med. 2018, 197, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Mascheroni, D.; Kolobow, T.; Fumagalli, R.; Moretti, M.P.; Chen, V.; Buckhold, D. Acute respiratory failure following pharmacologically induced hyperventilation: An experimental animal study. Intensive Care Med. 1988, 15, 8–14. [Google Scholar] [CrossRef]
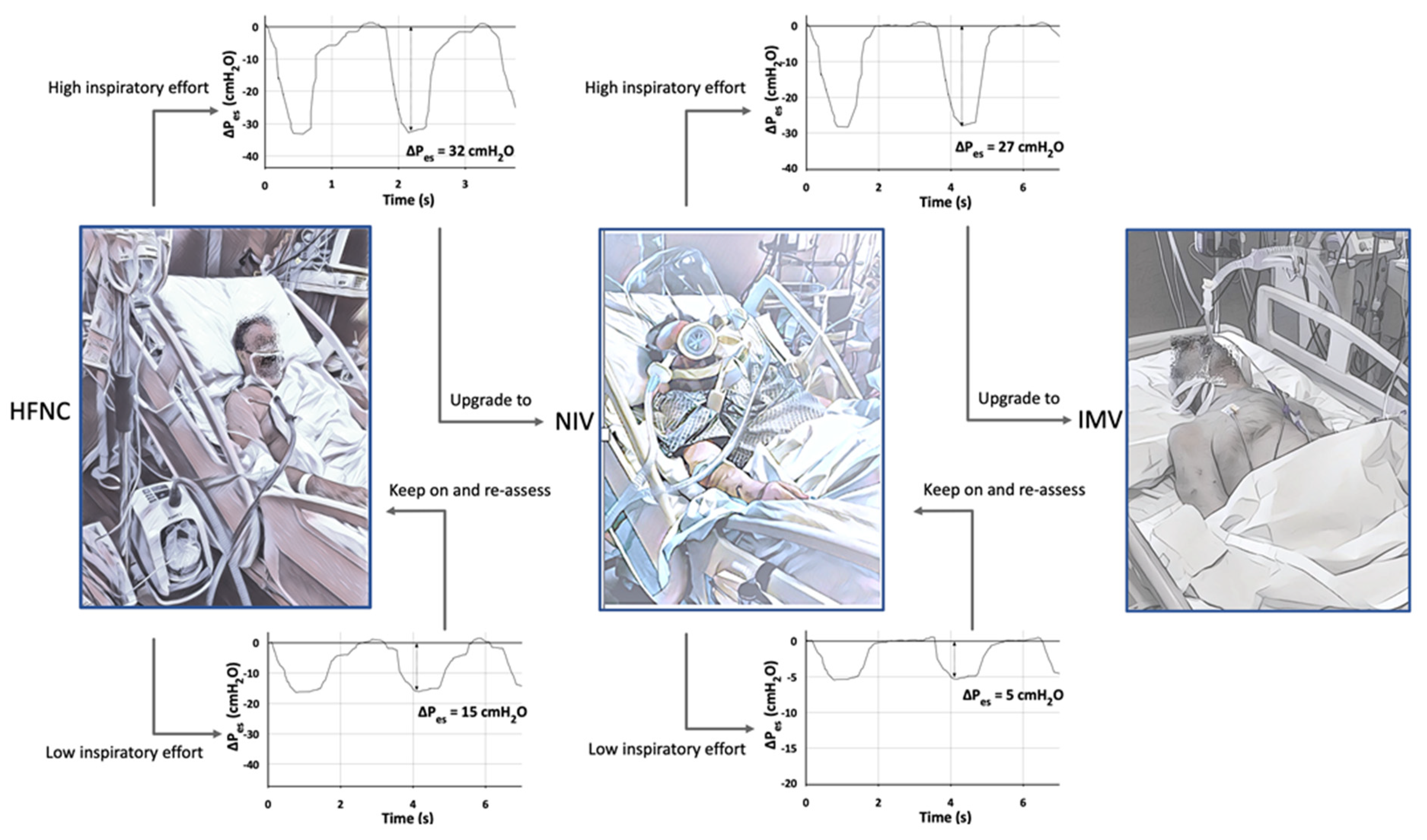
- Tonelli, R.; Fantini, R.; Tabbì, L.; Castaniere, I.; Pisani, L.; Pellegrino, M.R.; Della Casa, G.; D’Amico, R.; Girardis, M.; Nava, S.; et al. Early Inspiratory Effort Assessment by Esophageal Manometry Predicts Noninvasive Ventilation Outcome in De Novo Respiratory Failure. A Pilot Study. Am. J. Respir. Crit. Care Med. 2020, 202, 558–567. [Google Scholar] [CrossRef] [Green Version]
- Brochard, L.; Slutsky, A.; Pesenti, A. Mechanical Ventilation to Minimize Progression of Lung Injury in Acute Respiratory Failure. Am. J. Respir. Crit. Care Med. 2017, 195, 438–442. [Google Scholar] [CrossRef]
- Goligher, E.C.; Fan, E.; Herridge, M.S.; Murray, A.; Vorona, S.; Brace, D.; Rittayamai, N.; Lanys, A.; Tomlinson, G.; Singh, J.M.; et al. Evolution of Diaphragm Thickness during Mechanical Ventilation. Impact of Inspiratory Effort. Am. J. Respir. Crit. Care Med. 2015, 192, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, E.; Agostoni, E. Continuous recording of pleural surface pressure at various sites. Respir. Physiol. 1973, 19, 356–368. [Google Scholar] [CrossRef]
- Minh, V.D.; Friedman, P.J.; Kurihara, N.; Moser, K.M. Ipsilateral transpulmonary pressures during unilateral electrophrenic respiration. J. Appl. Physiol. 1974, 37, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Uchiyama, A.; Matsuura, N.; Mashimo, T.; Fujino, Y. The Comparison of Spontaneous Breathing and Muscle Paralysis in Two Different Severities of Experimental Lung Injury*. Crit. Care Med. 2013, 41, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Nakahashi, S.; Nakamura, M.A.M.; Koyama, Y.; Roldan, R.; Torsani, V.; De Santis, R.R.; Gomes, S.; Uchiyama, A.; Amato, M.B.P.; et al. Volume-controlled Ventilation Does Not Prevent Injurious Inflation during Spontaneous Effort. Am. J. Respir. Crit. Care Med. 2017, 196, 590–601. [Google Scholar] [CrossRef]
- Marini, J.J.; Hotchkiss, J.; Broccard, A.F. Bench-to-bedside review: Microvascular and airspace linkage in ventilator-induced lung injury. Crit. Care 2003, 7, 435–444. [Google Scholar] [CrossRef] [Green Version]
- Hooijman, P.E.; Beishuizen, A.; Witt, C.C.; de Waard, M.C.; Girbes, A.R.J.; Spoelstra-de Man, A.M.E.; Niessen, H.W.M.; Manders, E.; van Hees, H.W.H.; van den Brom, C.E.; et al. Diaphragm Muscle Fiber Weakness and Ubiquitin–Proteasome Activation in Critically Ill Patients. Am. J. Respir. Crit. Care Med. 2015, 191, 1126–1138. [Google Scholar] [CrossRef]
- Giustivi, D.; Bottazzini, F.; Belliato, M. Respiratory Monitoring at Bedside in COVID-19 Patients. J. Clin. Med. 2021, 10, 4943. [Google Scholar] [CrossRef]
- Xie, J.; Tong, Z.; Guan, X.; Du, B.; Qiu, H.; Slutsky, A.S. Critical care crisis and some recommendations during the COVID-19 epidemic in China. Intensive Care Med. 2020, 46, 837–840. [Google Scholar] [CrossRef] [Green Version]
- Guan, W.; Ni, Z.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Dhont, S.; Derom, E.; Van Braeckel, E.; Depuydt, P.; Lambrecht, B.N. The pathophysiology of ‘happy’ hypoxemia in COVID-19. Respir. Res. 2020, 21, 198. [Google Scholar] [CrossRef]
- Bertran Recasens, B.; Martinez-Llorens, J.M.; Rodriguez-Sevilla, J.J.; Rubio, M.A. Lack of dyspnea in patients with COVID-19: Another neurological conundrum? Eur. J. Neurol. 2020, 27, e40. [Google Scholar] [CrossRef]
- Tonelli, R.; Busani, S.; Tabbì, L.; Fantini, R.; Castaniere, I.; Biagioni, E.; Mussini, C.; Girardis, M.; Clini, E.; Marchioni, A. Inspiratory Effort and Lung Mechanics in Spontaneously Breathing Patients with Acute Respiratory Failure due to COVID-19: A Matched Control Study. Am. J. Respir. Crit. Care Med. 2021, 204, 725–728. [Google Scholar] [CrossRef]
- Coppola, S.; Pozzi, T.; Busana, M.; Bichi, F.; Camponetti, V.; Chiumello, D. Oesophageal manometry and gas exchange in patients with COVID-19 acute respiratory distress syndrome. Br. J. Anaesth. 2020, 125, e437–e438. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.L.; Ball, L.; Rocco, P.R.M.; Pelosi, P. Power to mechanical power to minimize ventilator-induced lung injury? Intensive Care Med. Exp. 2019, 7, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becher, T.; van der Staay, M.; Schädler, D.; Frerichs, I.; Weiler, N. Calculation of mechanical power for pressure-controlled ventilation. Intensive Care Med. 2019, 45, 1321–1323. [Google Scholar] [CrossRef] [PubMed]
- Esnault, P.; Cardinale, M.; Hraiech, S.; Goutorbe, P.; Baumstrack, K.; Prud’homme, E.; Bordes, J.; Forel, J.-M.; Meaudre, E.; Papazian, L.; et al. High Respiratory Drive and Excessive Respiratory Efforts Predict Relapse of Respiratory Failure in Critically Ill Patients with COVID-19. Am. J. Respir. Crit. Care Med. 2020, 202, 1173–1178. [Google Scholar] [CrossRef]
- Weaver, L.; Das, A.; Saffaran, S.; Yehya, N.; Scott, T.E.; Chikhani, M.; Laffey, J.G.; Hardman, J.G.; Camporota, L.; Bates, D.G. High risk of patient self-inflicted lung injury in COVID-19 with frequently encountered spontaneous breathing patterns: A computational modelling study. Ann. Intensive Care 2021, 11, 109. [Google Scholar] [CrossRef]
- Yoshida, T.; Brochard, L. Esophageal pressure monitoring. Curr. Opin. Crit. Care 2018, 24, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Marini, J.J.; Gattinoni, L. Management of COVID-19 Respiratory Distress. JAMA 2020, 323, 2329. [Google Scholar] [CrossRef]
- Akoumianaki, E.; Maggiore, S.M.; Valenza, F.; Bellani, G.; Jubran, A.; Loring, S.H.; Pelosi, P.; Talmor, D.; Grasso, S.; Chiumello, D.; et al. The Application of Esophageal Pressure Measurement in Patients with Respiratory Failure. Am. J. Respir. Crit. Care Med. 2014, 189, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Mojoli, F.; Torriglia, F.; Orlando, A.; Bianchi, I.; Arisi, E.; Pozzi, M. Technical aspects of bedside respiratory monitoring of transpulmonary pressure. Ann. Transl. Med. 2018, 6, 377. [Google Scholar] [CrossRef] [PubMed]
- Walamies, M.A. Perception of esophageal manometry. Dis. Esophagus 2002, 15, 46–49. [Google Scholar] [CrossRef] [PubMed]

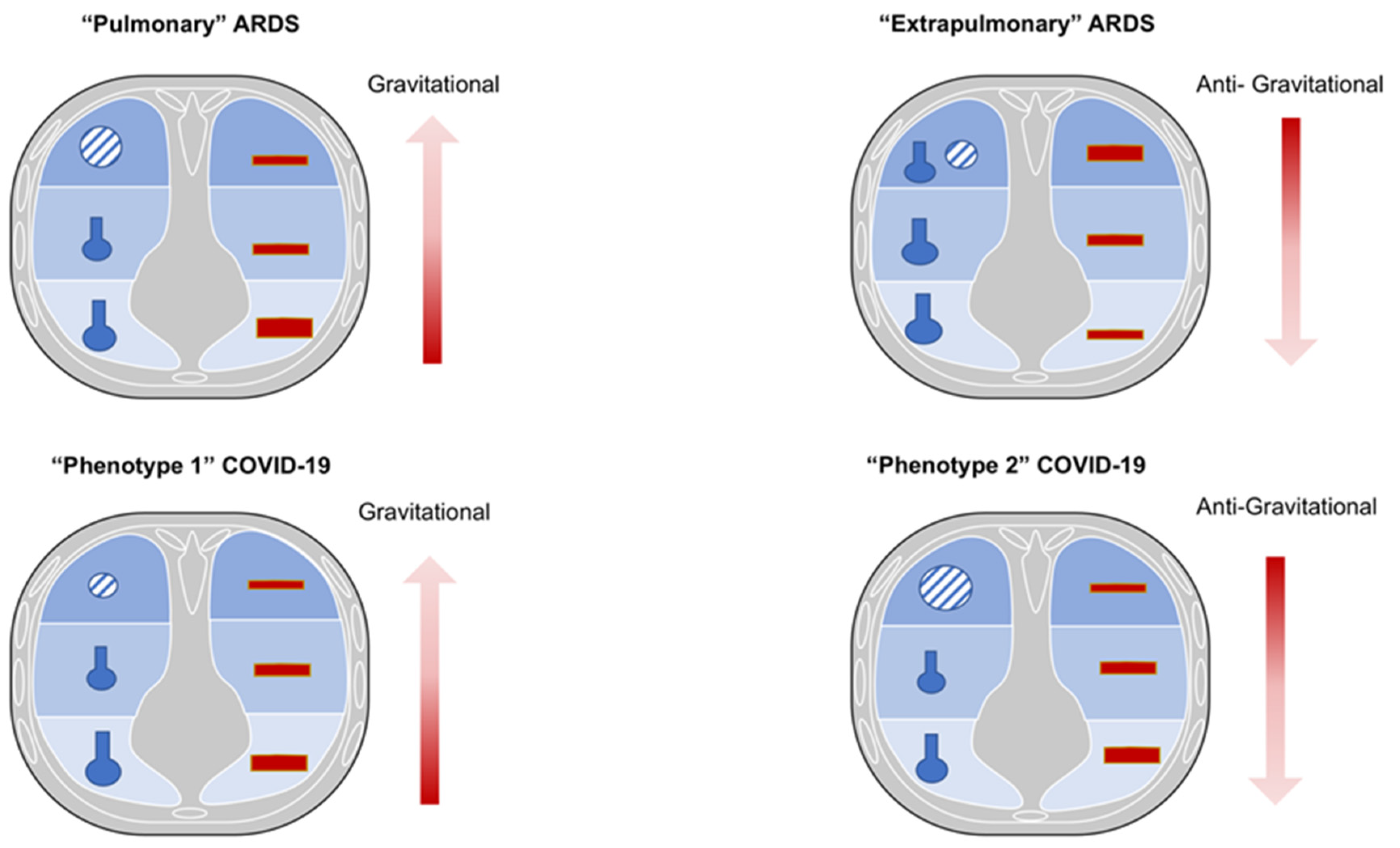


| Disease Type | Histopathological Findings | Radiological Findings | Clinical Characteristics and Management | |
|---|---|---|---|---|
| Non-COVID-ARDS (DAD) | “Pulmonary” ARDS | Alveolar epithelial damage and collapse Neutrophilic infiltrates in terminal bronchioles and surrounding alveoli with confluence of infiltrates between adjacent lobules (prevalent) Fibrinous exudates in alveoli Interstitial edema (rare) Increased collage in the interstitial space Altered type I and II pneumocytes with nuclear atypia and hyperplasia (necrosis type I cells, proliferation type II cells) Apoptotic neutrophils (prevalent) Normal capillary endothelium Mallory like inclusions in type II pneumocytes Proliferation of fibroblast and myofibroblast Possible DAD (hyaline membrane plus intra-alveolar edema, necrosis of alveolar type I cells, proliferation of alveolar type II cells, interstitial proliferation of fibroblast and myofibroblast, interstitial fibrosis) | Asymmetric consolidation and ground-glass opacification Possible pleural effusion and emphysema Predominance of air bronchograms and pneumomediastinum | Clinical manifestation Hypoxia due to low V/Q and true shunt lung areas Suggested treatment IMV (low VT 4–6 mL/kg PBW, higher PEEP, low Pplat < 28–30 cmH2O, ΔP < 13 cmH2O) Prone position (to redistribute pulmonary blood flow from dorsal to ventral lung regions, decreased CO2 washout, no improvement in regional alveolar ventilation) |
| “Extrapulmonary” ARDS | Alveolar epithelial damage and collapse Neutrophilic infiltrates (rare) Fibrinous exudates in alveoli (rare) Interstitial edema (prevalent) Increased collage in the interstitial space Normal type I and II pneumocytes Apoptotic neutrophils (rare) Damaged capillary endothelium Possible DAD (hyaline membrane plus intra-alveolar edema, necrosis of alveolar type I cells, proliferation of alveolar type II cells, interstitial proliferation of fibroblast and myofibroblast, interstitial fibrosis) | Symmetric ground-glass consolidation (mainly distributed in the middle-basal levels and vertebral position) and opacification (greater in the central third of the lung than in the sternal or vertebral third without significant craniocaudal predominance) Possible pleural effusion and emphysema | Clinical manifestation Hypoxia due to alveolar collapse of dependent lung regions with gravitational distribution of perfusion and true shunt lung areas Suggested treatment IMV (low VT 4–6 mL/kg PBW, higher PEEP, low Pplat < 28–30 cmH2O, ΔP < 13 cmH2O) Prone position (recruitment of collapsed areas maintaining higher perfusion toward dorsal lung regions, increased CO2 washout, improvement in regional alveolar ventilation) | |
| COVID-19 ARDS | Phenotype 1 | Alveolar collapse and rupture Intra-alveolar hemorrhage Hyaline tissue formation (rare) Microthrombi, vasculitis or vascular thrombosis Polymorphonuclear and monocytes infiltration (initial) SARS-CoV-2 replication in type II pneumocytes Reactive pneumocytes with nuclear atypia and hyperplasia Mallory like intracytoplasmic inclusions in type II pneumocytes Masson’s bodies | Multiple focal over perfused ground glass opacities and normally aerated areas Possible diversion of ventilation toward non-dependent aerated lung regions and reduction in pulmonary perfusion due to increased airway pressure Collapse of capillaries and/or micro-thrombosis and formation of no recruitable atelectasis | Clinical manifestation Normal compliance of the respiratory system Hypoxia (increased areas with altered V/Q ratio) Suggested treatment NIRS (HFNC, CPAP, NIV) with high FiO2 and respiratory monitoring (i.e., clinical deterioration, gas exchange, FOT, esophageal manometry) IMV when NIRS failed (using lower PEEP) |
| Phenotype 2 | Alveolar collapse and rupture Intra-alveolar hemorrhage Hyaline tissue formation (prevalent) Microthrombi, vasculitis or vascular thrombosis Early fibroblastic interstitial fibrosis, septal and para-septal reparative fibrosis Polymorphonuclear and monocytes infiltration (prevalent) Reactive pneumocytes with nuclear atypia and hyperplasia Mallory like intracytoplasmic inclusions in type II pneumocytes Masson’s bodies | Patchy ARDS-like pattern Inhomogeneously distributed and hyper/hypo-perfused areas Increased lung weight and consolidated Non-aerated lung regions (dependent lung regions) | Clinical manifestations Decrease of aerated lung regions Impairment of compliance of the respiratory system Increased shunt (blood flow redistribution to injured areas with hypoxic vasoconstriction, thrombosis, and compression of capillaries) Suggested treatment IMV (low VT 4–6 mL/kg PBW, higher PEEP to redistribute ventilation and perfusion, Prone positioning (partial redistribution from dorsal to ventral areas, no effective recruitment) | |
| Phenotype 3/F | Hyaline membranes Fibroblastic interstitial fibrosis, septal and para-septal reparative fibrosis (prevalent) Parenchymal bands, irregular interfaces, reticular opacities Traction bronchiectasis with or without honeycombing | Final evolution to fibrosis Possible traction bronchiectasis and reticulation | Clinical manifestations Low diffusing capacity for carbon monoxide (DLCO), altered gas exchange Suggested treatment Symptomatic treatment (i.e., oxygen, corticosteroids, antifibrotic drugs like nintedanib, antibiotics, etc.) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelosi, P.; Tonelli, R.; Torregiani, C.; Baratella, E.; Confalonieri, M.; Battaglini, D.; Marchioni, A.; Confalonieri, P.; Clini, E.; Salton, F.; et al. Different Methods to Improve the Monitoring of Noninvasive Respiratory Support of Patients with Severe Pneumonia/ARDS Due to COVID-19: An Update. J. Clin. Med. 2022, 11, 1704. https://doi.org/10.3390/jcm11061704
Pelosi P, Tonelli R, Torregiani C, Baratella E, Confalonieri M, Battaglini D, Marchioni A, Confalonieri P, Clini E, Salton F, et al. Different Methods to Improve the Monitoring of Noninvasive Respiratory Support of Patients with Severe Pneumonia/ARDS Due to COVID-19: An Update. Journal of Clinical Medicine. 2022; 11(6):1704. https://doi.org/10.3390/jcm11061704
Chicago/Turabian StylePelosi, Paolo, Roberto Tonelli, Chiara Torregiani, Elisa Baratella, Marco Confalonieri, Denise Battaglini, Alessandro Marchioni, Paola Confalonieri, Enrico Clini, Francesco Salton, and et al. 2022. "Different Methods to Improve the Monitoring of Noninvasive Respiratory Support of Patients with Severe Pneumonia/ARDS Due to COVID-19: An Update" Journal of Clinical Medicine 11, no. 6: 1704. https://doi.org/10.3390/jcm11061704
APA StylePelosi, P., Tonelli, R., Torregiani, C., Baratella, E., Confalonieri, M., Battaglini, D., Marchioni, A., Confalonieri, P., Clini, E., Salton, F., & Ruaro, B. (2022). Different Methods to Improve the Monitoring of Noninvasive Respiratory Support of Patients with Severe Pneumonia/ARDS Due to COVID-19: An Update. Journal of Clinical Medicine, 11(6), 1704. https://doi.org/10.3390/jcm11061704












